Effects of Pressure on Immersed Objects
The effects of pressure on the air in a cylinder open at its bottom end and lowered to various depths in water. If the cylinder is filled with atmospheric air at the surface, and if no air is supplied to the cylinder on its way down, the volume and density of air in the cylinder will follow Boyle’s Law as the pressure of the water increases.
Relationship Between Depth, Pressure, and Volume
At the surface, the pressure of air in the cylinder is 1 bar. When the cylinder is lowered to a depth of 10 m, the water exerts an additional pressure of 1 bar, so the total absolute pressure on the air in the cylinder is 2 bar. As a result, the volume of air in the cylinder is reduced to one-half of what it was at the surface and the density of the air is doubled. At 20 m the water exerts a pressure of 2 bar and the absolute pressure in the cylinder is 3 bar, so the volume of the air will be only a third of what it was at the surface and the density will be trebled. At a depth of 90 m the water is exerting a pressure of 9 bar and the absolute pressure in the cylinder is 10 bar; the air volume is now only a tenth of its surface volume and its density is ten times as great.

It should be noticed that the changes in volume relative to pressure are much greater near the surface, e.g. for the change in pressure of 1 bar from the surface to a depth of 10 m the volume of air is reduced by half whereas a change of pressure of 1 bar in descending from 30 m to 40 m reduced the volume from a quarter to a fifth, a reduction of only 1/20 (5%) of its surface volume. This change in volume for each bar of pressure becomes smaller the deeper the cylinder goes. The volume will expand correspondingly as the cylinder is brought to the surface.
It must be remembered that throughout the changes in volume, the actual amount or the ‘mass’ of the air in the cylinder remains the same – none has escaped – and the volume change is affected only by a corresponding change in the density of the air, caused by compression.
In general principle, the effect of water pressure on different small objects submerged to a depth of 1 bar. A solid ball would have pressure acting upon it with equal force in all directions and would not change in shape or size. A hollow metal sphere filled with air at atmospheric pressure would also remain unaffected and the air inside would remain at atmospheric pressure, provided the shell of the sphere was strong enough to withstand the external pressure.
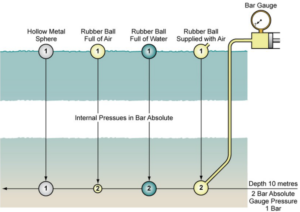
A soft rubber ball full of air at atmospheric pressure at the surface would, however, be contracted by the water pressure as it descended, compressing the air inside to the surrounding water pressure. Thus, at a depth of 10 m, the ball would be only half its original size and the air inside would be at 2 bar absolute pressure. The ball would of course recover its original size and internal pressure as it was brought back to the surface. The same ball could be prevented from contracting by supplying it with air from the surface at a pressure equal to the surrounding water pressure.
The air pressure would have to be correspondingly reduced as the ball was brought to the surface or it would increase in size and probably burst. If the ball were filled with water it would retain its original size and shape whatever its depth, as water is, for all practical purposes, incompressible, and the water would always be at the same absolute pressure as the surrounding water.
Effect of Pressure on Immersed Objects
When considering larger objects, the difference in pressure between the bottom and top of the object must be considered; for instance, a diver 1.8 m tall and standing up underwater will have a difference in pressure between their head and their feet of approximately 0.18 bar. The effect of water pressure on a large rubber ball filled with air at atmospheric pressure at the surface would be to contact the bottom of the ball to a greater extent than the top:

it is important to remember, however, that whatever shape the ball assumed Boyle’s Law would still apply, i.e. if the pressure were doubled by the contraction, the volume of the air space would be halved. It should also be realized that the internal air pressure will, for all practical purposes, be uniform throughout the ball as the weight of air is negligible. However, if the air is replaced by water the ball will not change in shape or size, but because of the weight of water in the ball, the pressure at the bottom of the ball will be greater than that at the top.
Conclusion:
In conclusion, Pressure has significant effects on immersed objects in water. Boyle’s law governs the relationship between pressure and volume of air in a cylinder. As depth increases, volume decreases, and density increases. The changes in volume are more pronounced near the surface compared to a deeper depth.
The mass of air remains constant and volume changes are due to compression. Different objects respond differently to water pressure. Solid balls and hollow metal spheres remain unaffected, while soft rubber balls compress. Water pressure does not affect a ball filled with water. Larger objects experience varying pressure differences. Boyle’s Law still applies, and the volume of air space changes accordingly. Understanding these effects is crucial for underwater exploration and safety.

